Copyright (c) 2010 Sergey Bilyk.
Published on April 18, 2010.
License: public domain.
1. Introduction
WARNING:
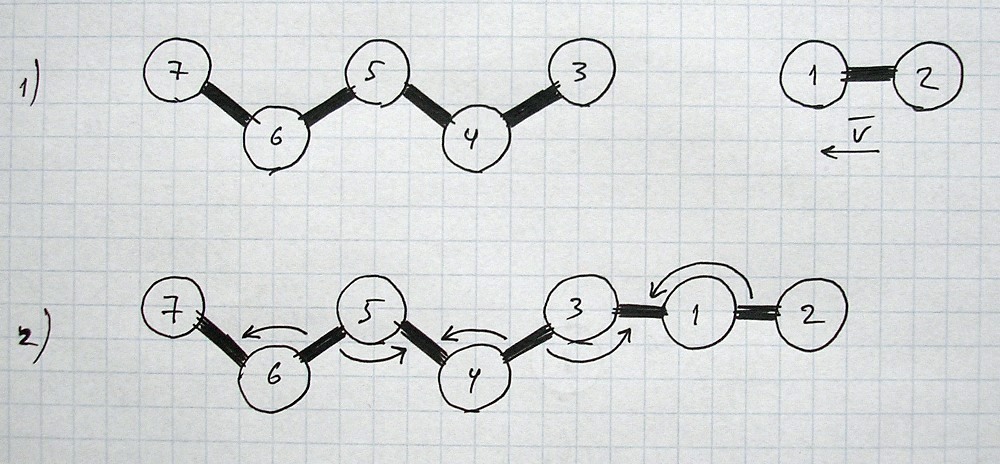
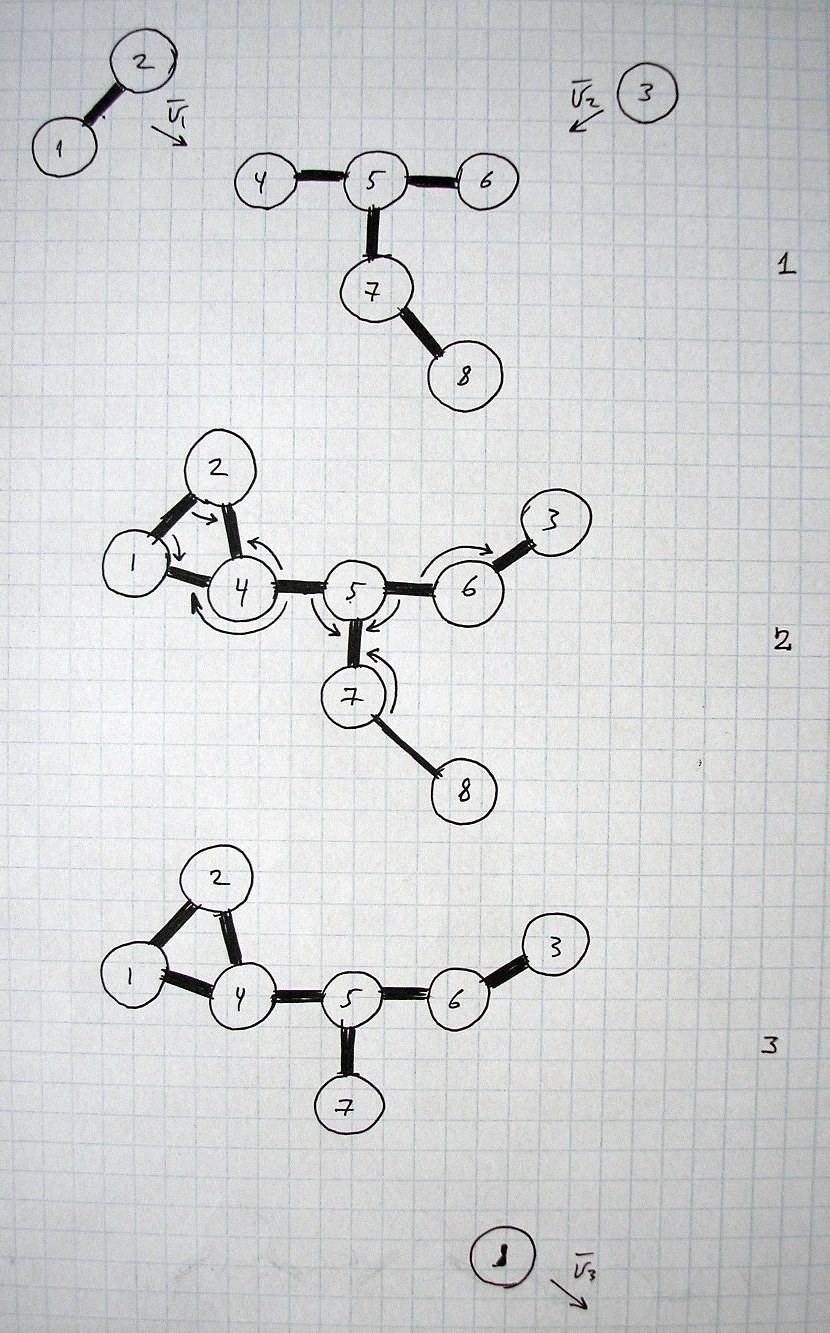
The explanation of chemical bonds and interactions below is not complete.This is just a rough general overview to show some approximate image of part of reality in this area and conditions.
This is just a part of the laws/processes that take place during chemical reactions.
It may be considered as a special case (of more general and uniform processes) characteristic for the conditions and states of space/matter and other parameters that we have at earth and at the nearest surroundings.
For other conditions (or with change of basic parameters) the result (general image of observable states and interactions) may differ very significantly (actually this is one of the reasons why modern science cannot explain much beyond the conditions we have at earth).
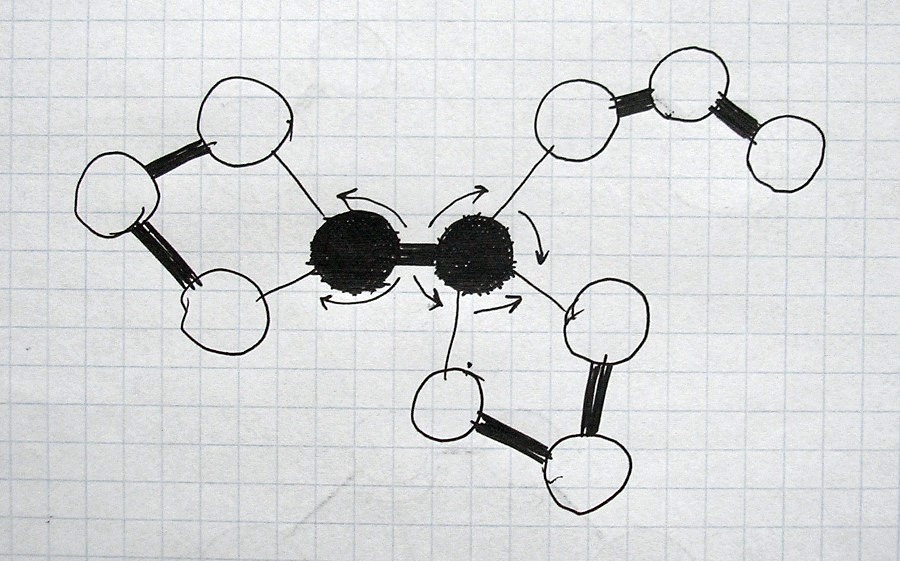
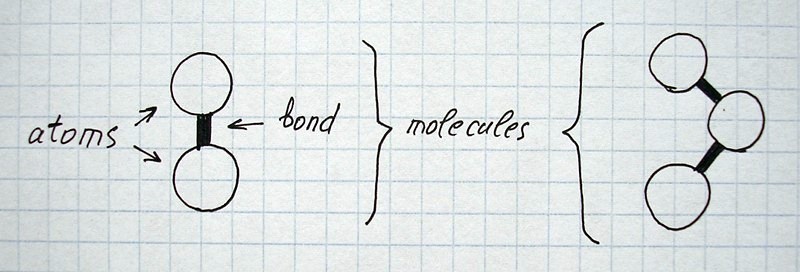
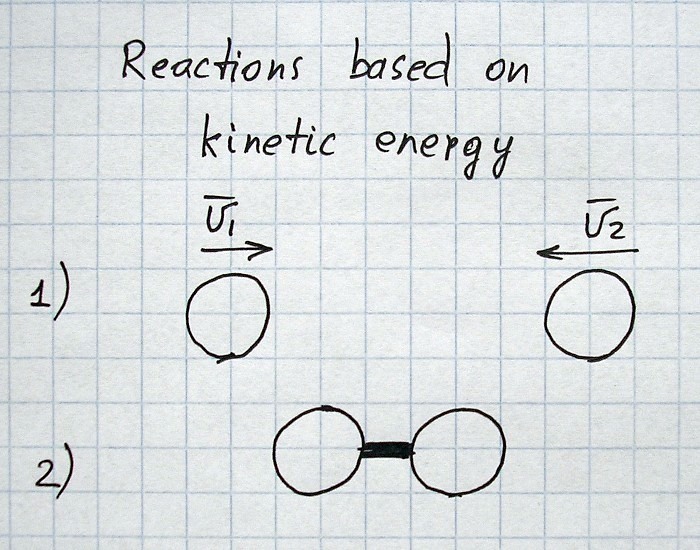
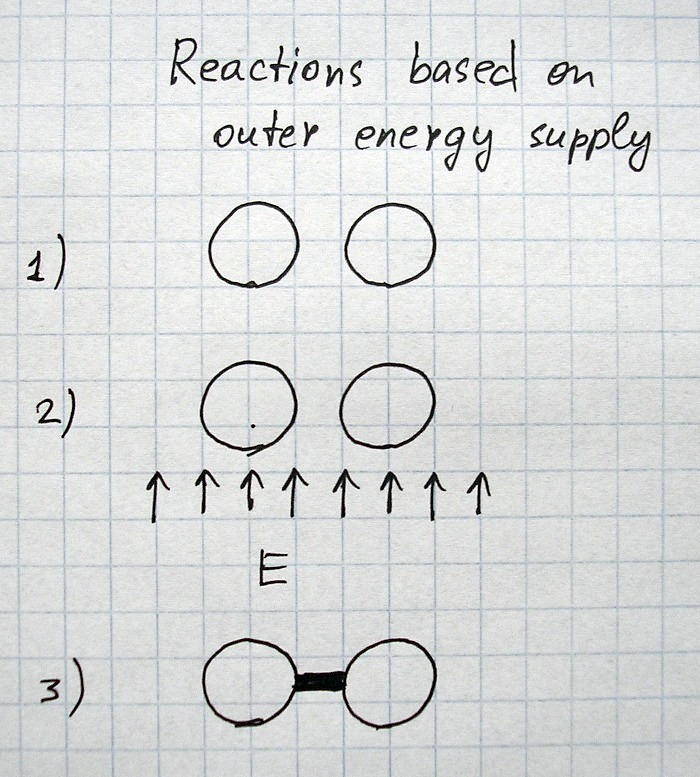
All images and explanations are exclusively schematic and do not correspond to concrete atoms or specific states of other things/substances.
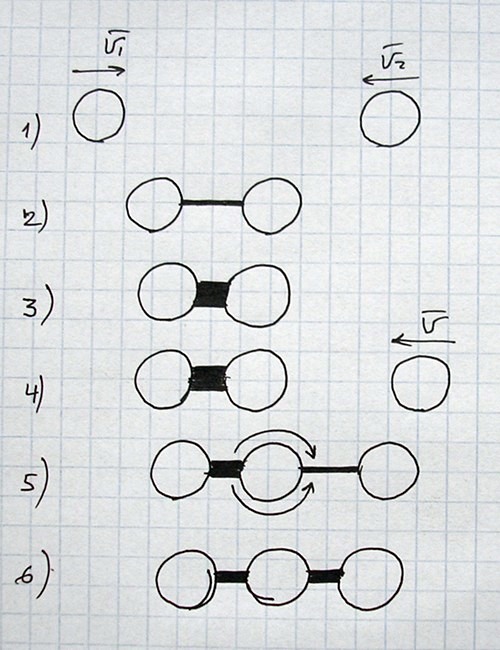
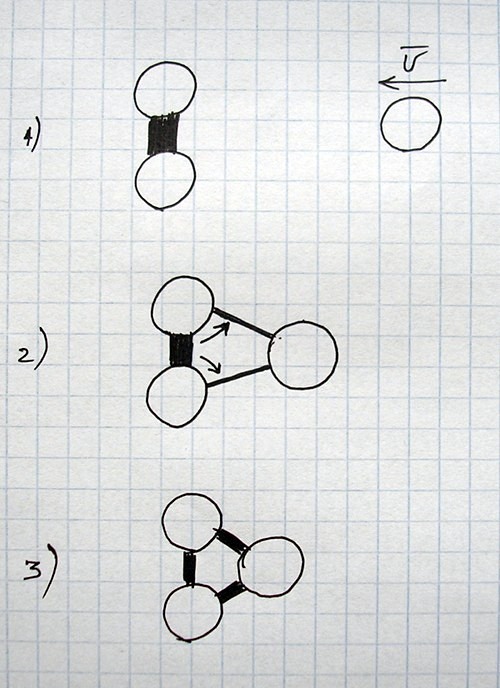
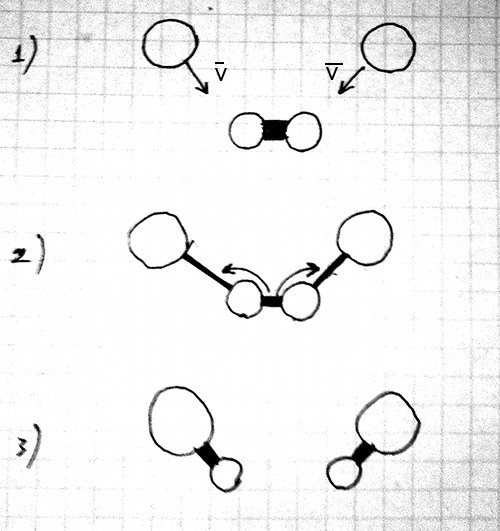
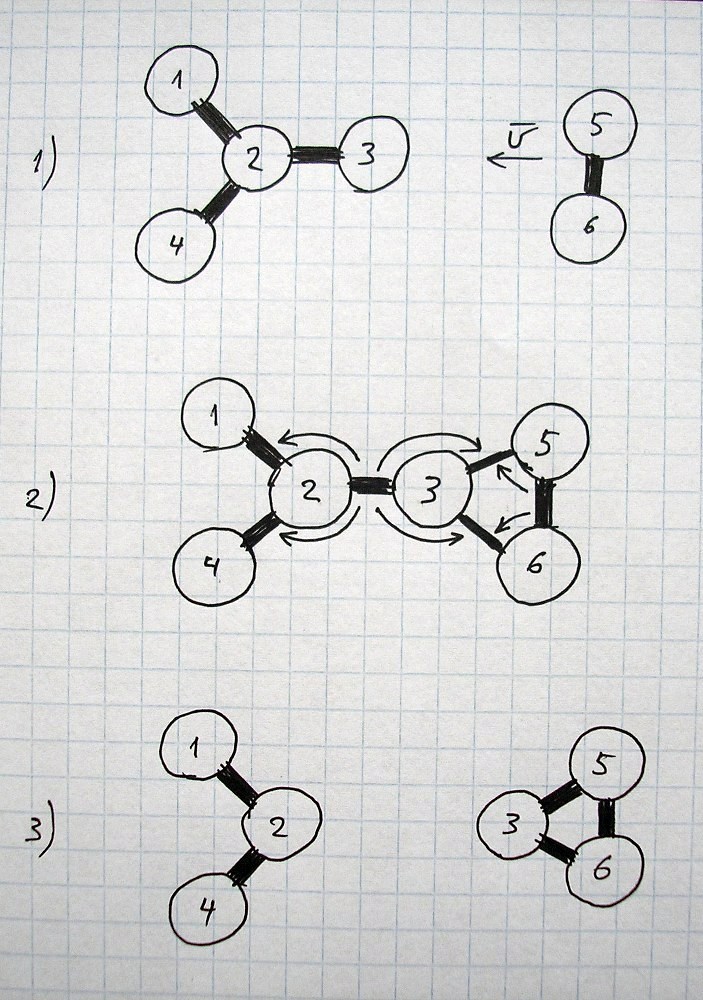



| Energy or state of material sustained mainly by corresponding kind of energy | Main dependencies of force for current conditions (for two objects) |
| Gravitational | F=f(m1, m2, R, ...) |
| Electricity | F=f(q1, q2, R, ...) |
| Chemical, crystalline, amorphous, liquid, ... | F=f(x1, x2, R, ...) |